Abstract
BACKGROUND
Multiple myeloma (MM) is a relapsing haematologic cancer that remains incurable. For patients aged < 65 years or fit patients < 70 years, autologous stem cell transplant (ASCT) is the gold standard first-line treatment. Treatment guidelines recommend lenalidomide (LEN), the only approved agent, as maintenance therapy (MT) post-ASCT (NCCN Clinical Practice Guidelines. Multiple myeloma. V3.2017; Moreau P, et al . Ann Oncol . 2017 Apr 27. [Epub ahead of print]). Post-progression, in the relapsed/refractory setting, a number of newer agents and combination treatments have been approved. While all these developments benefit patients with MM, there is a potential impact on National Health Service budget. Economic information can therefore play an important role in market access decision-making for innovative medicines.
OBJECTIVE
This analysis examines the cost impact of LEN as MT post-ASCT in the context of an evolving and complex treatment pathway from the perspective of the EU5 health systems (ie, France, Germany, Italy, Spain, and the United Kingdom).
METHODS
A cost-pathway model was developed to compare direct costs over 5 years following ASCT from the perspective of national healthcare providers in the EU5. The model follows a cohort of patients from completion of ASCT through MT or no MT and up to 2 lines of therapy following disease progression after ASCT. LEN MT was assumed to be given at a dose of 10 mg with 50% of patients on 28/28-day dosing and another 50% on 21/28-day dosing. This reflects dosing in the Myeloma XI trial and clinical prescribing in the real world. Duration of therapy was based on the CALGB study 100104 (McCarthy PL, et al. N Engl J Med. 2012;366:1770-81). Following progression in the model, patients were allocated to the following treatments based on expert clinical opinion and assuming that all newer agents were relatively accessible in the clinic: LEN/dexamethasone (Rd), bortezomib/dexamethasone (Vd), carfilzomib/Rd, carfilzomib/dexamethasone, ixazomib/Rd, daratumumab/Rd, daratumumab/Vd, or elotuzumab/Rd. Costs considered included drug acquisition costs (at list price or public prices), administration costs, and costs for management of adverse events. Two scenarios were assessed: 1) LEN MT, in which 80% of eligible patients receive LEN therapy and 2) no MT, in which only 20% of patients receive LEN therapy. Extreme scenarios corresponding to 100% and 0% of patients receiving LEN MT were also assessed.
RESULTS
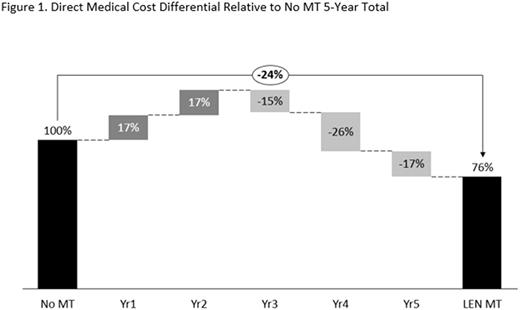
In the base case, direct medical costs per patient for LEN MT were €209,600 over the 5-year period compared with €276,900 for the no MT treatment strategy. In the no MT scenario, annual costs increased from €16,000 in years 1 and 2 to €81,200 in year 5. Conversely, in the LEN MT scenario, annual costs decreased from €62,600 in years 1 and 2 to €24,400-€34,400 for years 3-5. The increase in costs in years 3-5 in the no MT scenario reflected costs for further lines of treatment. The reduction in costs for years 3-5 in the LEN MT scenario reflected the benefits of LEN MT resulting in a prolonged disease-free period. LEN MT direct medical costs over a 5-year period were therefore 24% less than those for no MT (Figure 1). In the extreme scenarios, direct medical costs per patient over 5 years were €187,200 (100% LEN MT) and €299,400 (0% LEN MT).
DISCUSSION
The results of this cost-pathway analysis suggest that LEN MT reduces the overall direct medical costs over the first 5 years post-ASCT for the management of eligible patients with MM in the context of an evolving and complex treatment pathway. Furthermore, use of LEN MT spreads the cost of management of these patients more evenly across the 5 years post-ASCT, thus easing the economic burden for the healthcare system. This analysis did not consider resource savings that are also likely to be achieved with LEN MT (Ashcroft J, et al. EHA 2017. [abstract E1463]) and may thus be a conservative estimate of the likely cost savings. Real-world cost studies are warranted to investigate further the impact on costs and resource use of the uptake of LEN MT to ensure that patients are able to benefit from the improved outcomes that access to LEN MT can provide.
Jackson: Chugai: Honoraria; Celgene: Honoraria; J&J: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Dutton: Celgene: Consultancy. Hughes: Celgene: Consultancy. Dhanasiri: Celgene Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.